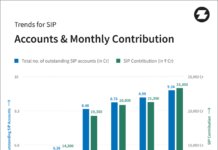
TAMPA, Fla., Sept. 17, 2022 /PRNewswire/ — The US FDA has granted an Investigational Device Exemption (IDE) approval for MagicTouch Sirolimus Coated Balloon (SCB) indicated for In-Stent Restenosis (ISR).
US FDA’s IDE approval permits the MagicTouch SCB for use in a pivotal scientific research to help security and effectiveness of this mix product. The information generated from this IDE scientific research will help a pre-market approval (PMA) utility within the USA.
MagicTouch SCB is the world’s first Sirolimus-coated Balloon with in depth business utilization in Europe, main markets of Asia and the Mid-Eastern markets. More than 100 thousand sufferers have been handled with MagicTouch SCB in these markets.
About MagicTouch SCB:
MagicTouch SCB is a CE marked and commercially marketed Sirolimus coated balloon developed by Concept Medical, utilizing proprietary Nanoluté Technology. MagicTouch SCB has been utilized in >50,000 sufferers in main international markets.
About Concept Medical Inc (CMI):
CMI is headquartered in Tampa, Florida and has operational workplaces in The Netherlands, Singapore and Brazil and manufacturing models in India. CMI makes a speciality of growing drug-delivery techniques and has distinctive and patented expertise platforms that may be deployed to ship any drug / pharmaceutical agent throughout the luminal surfaces of blood vessels.
www.conceptmedical.com
Photo – https://mma.prnewswire.com/media/1901255/MagicTouch.jpg
Logo – https://mma.prnewswire.com/media/1244676/Concept_Medical_Logo.jpg

![]() View authentic content material:https://www.prnewswire.co.uk/news-releases/magictouch-scb-receives-ide-approval-for-in-stent-restenosis-indication-301626647.html
View authentic content material:https://www.prnewswire.co.uk/news-releases/magictouch-scb-receives-ide-approval-for-in-stent-restenosis-indication-301626647.html